GSK’s 5-in-1 meningococcal vaccine, Penmenvy (Meningococcal Groups A, B, C, W, and Y Vaccine) received U.S. Food and Drug Administration approval for use in individuals aged 10 through 25 years.
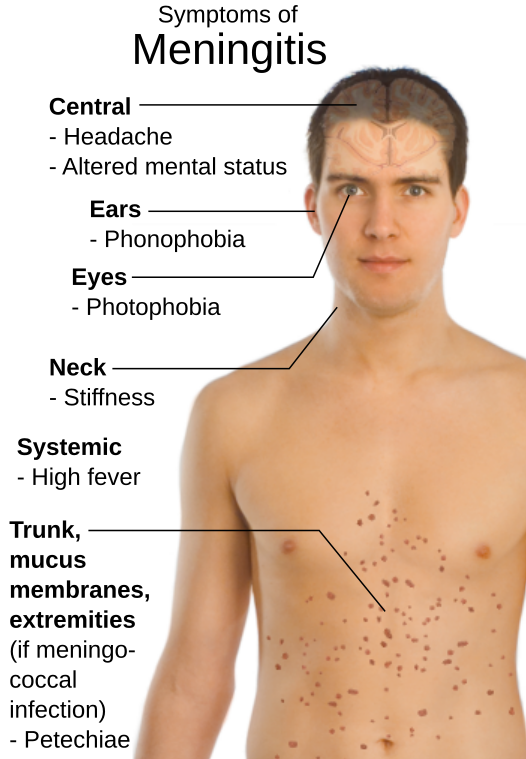
The vaccine targets five major serogroups of Neisseria meningitidis (A, B, C, W, and Y) which commonly cause invasive meningococcal disease (IMD).
The vaccine combines the antigenic components of GSK’s two well-established meningococcal vaccines, BEXSERO (Meningococcal Group B Vaccine) and MENVEO (Meningococcal [Groups A, C, Y, and W-135] Oligosaccharide Diphtheria CRM197 Conjugate Vaccine).
Tony Wood, Chief Scientific Officer, GSK, said: “We are excited about the opportunities ahead to help improve meningococcal vaccination coverage in the United States, especially for IMD caused by serogroup B. Building on our global leadership in meningococcal vaccination and our longstanding commitment to address unmet need in disease prevention, we aim to help protect more teens and young adults at a life stage when they are at an increased risk.”
IMD is an uncommon but serious illness that can lead to death for up to one in six of those who contract it in as little as 24 hours from onset, despite treatment.
Approximately one in five survivors may experience long-term consequences such as brain damage, amputations, hearing loss, and nervous system problems. Although anyone can get IMD, adolescents and young adults between the ages of 16 and 23 years are one of the groups at highest risk due to common behaviors that help transmit the bacteria that cause IMD (Neisseria meningitidis) such as living in close quarters like college dormitories, kissing and sharing drinks, utensils, or smoking devices.
Meningococcal meningitis with Rodney Rohde, PhD
The Centers for Disease Control and Prevention (CDC) reports in 2023, 438 confirmed and probable cases were reported. This is the largest number of U.S. meningococcal disease cases reported since 2013. Neisseria meningitidis serogroup Y drives much of this recent increase.